Use Chemical Equation to Describe Double Decomposition Reaction
One of the first practical batteries was invented by the British chemist. Fill in the boxes at the top of this page with your name centre number and candidate numbert t Answer all questions.

Balancing Chemical Equations Conservation Of Mass Worksheets And Labs Conservation Of Mass Chemistry Worksheets Chemical Equation
The rates at which reactants are consumed and products are formed during chemical reactions vary greatly.

. You have been warned. Most of the granular polymer is formed in the gas phase. To describe how the rate of a second-order reaction changes with concentration of reactants or products the differential derivative rate equation is used as well as the integrated rate equation.
When predicting the products for a reaction it is important to remember that atoms or ions will only combine in ways that make them stable otherwise the reaction will not happen under normal conditions. I Combination reaction ii Double displacement reaction Precipitation reaction iii Decomposition reaction. The differential rate law can show us how the rate of the reaction changes in time while the integrated rate equation shows how the concentration of species changes over time.
Your teacher is aware of this and on a multiple choice test will provide the answer arrived at by making this mistake. 1CR Thursday 14 May 2015 Morning Time. The ChemTeam has seen lots of students go right ahead and solve using the unbalanced equation supplied in the problem or test question for that matter.
2Fes 6HClaq -- 2FeCl3 aq 3H2g Write a balanced. The chemical nature of the reacting substances the state of subdivision one large. To use the chemical reaction calculator follow these steps.
A short summary of this paper. The reaction rate with a large E_a increases rapidly with increasing. We recommend using the latest version of Chrome Firefox Safari or Edge.
He formulated the following equation to describe the reaction. Balance the following equation and classify it as a combination decomposition single replacement or double replacement reaction. Write a balanced chemical equation for the decomposition reaction.
35 Full PDFs related to this paper. Chemists use their knowledge of synthesis decomposition single replacement and double replacement to predict what will happen in chemical reactions. Write a balanced chemical equation and use the necessary symbols from Table 111 to describe the reaction completely.
To break down mercury II oxide cinnabar into its individual elements for his oxygen. DONT use the same molar mass in steps two and four. Write the chemical equation of the reaction in which the following changes have taken place with an example of each.
I Change in colour. E_a indicates the sensitivity of the reaction to changes in temperature. Full PDF Package Download Full PDF Package.
Equation ref1453 is known as the Arrhenius equation and summarizes the collision model of chemical kinetics where T is the absolute temperature in K and R is the ideal gas constant 8314 JKmol. 6A Name the type of chemical reaction represented by the following equation. Bubbles of hydrogen gas and aqueous iron III chloride are produced when metallic iron is dropped into hydrochloric acid.
Answer the questions in the spaces provided t there may be. The reactions are carried out at temperature up to 90C 24. Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential as a measurable and quantitative phenomenon and identifiable chemical change with either electrical potential as an outcome of a particular chemical change or vice versaThese reactions involve electrons moving between electrodes via an electronically.
A peroxide initiator may be used and the reaction carried out in an aqueous medium with vigorous stirring. Describe the effects of chemical nature physical state temperature concentration and catalysis on reaction rates. 2 hours 4CH01CR 4SC01CR You must have.
Double check the equation. This means that it is. Download Full PDF Package.
Elements of Chemical Reaction Engineering Fifth Edition. 2HgO_s longrightarrow 2Hg_l O_2_g Single Replacement Reactions. One method of producing the latter involves the use of a 01 aqueous disuccinic acid peroxide solution.
We can identify five factors that affect the rates of chemical reactions. Ruler Calculator Instructions tt Use black ink or ball-point pen. The use of a double-arrow in the equation above denotes the partial reaction aspect of this process a concept addressed fully in the chapters on chemical equilibrium Figure 46 a Fruits such as oranges lemons and grapefruit contain the weak acid citric acid.
Science Double Award 4SC0 Paper. The PhET website does not support your browser.

Lesson Plan Bundle Chemical Reactions Distance Learning Chemical Reactions Chemistry Classroom Lesson

Predicting Products Of Synthesis And Decomposition Reactions Teaching Chemistry Scientific Method Lesson Writing Linear Equations

Types Of Chemical Reactions Binary Decomposition Decomposition Of A Binary Compound Contains Exactl Chemistry Lessons Teaching Chemistry Chemistry Education

Double Replacement Reaction Definition And Examples Reactions Covalent Bonding Ap Chemistry

Balance And Classify Five Types Of Chemical Reactions Synthesis Decomposition Single Replacement Double Replacement A Chemical Equation Equations Chemical

Chemical Reactions Types Definitions And Examples Teaching Chemistry Chemistry Classroom Chemistry Notes

What Is A Decomposition Reaction Definition And Examples Reactions Chemical Bond Ap Chemistry

Types Of Chemical Reactions Chemistry Classroom Teaching Chemistry Science Lessons
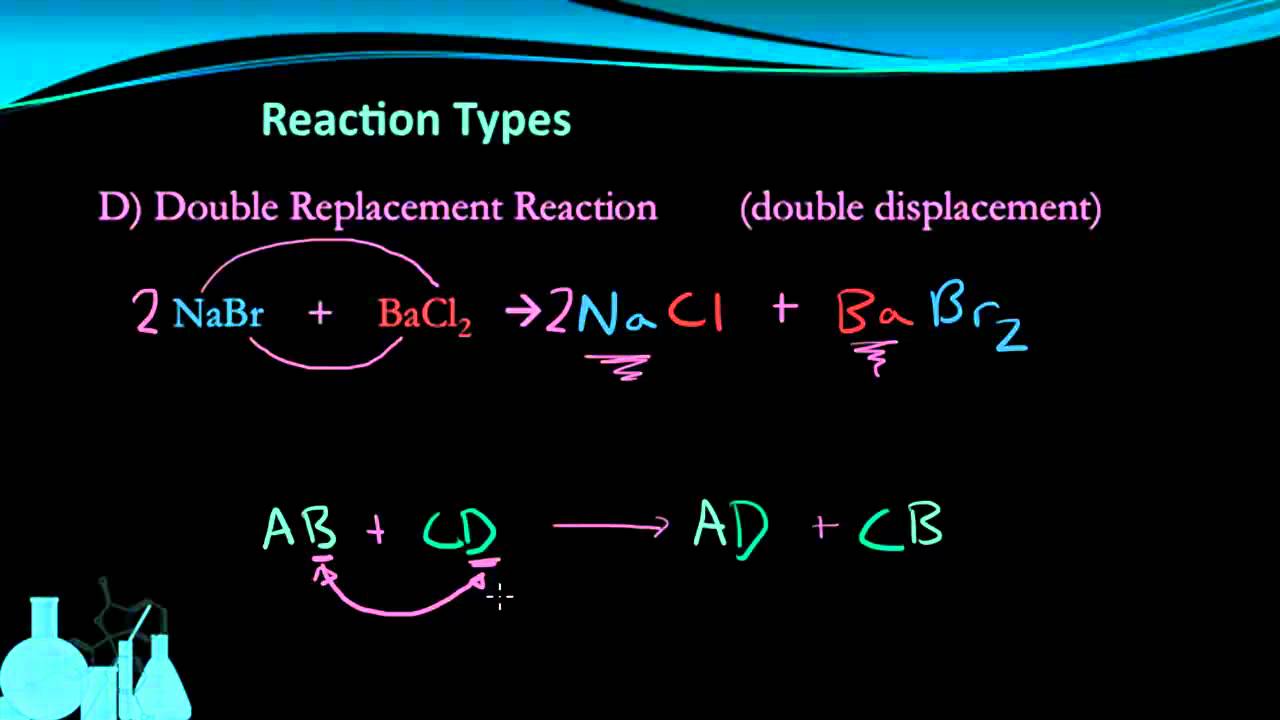
Chemistry Module 5 Types Of Chemical Reactions Http Www Youtube Com Watch V Aawccqb75d0 Single

Representation Of Four Basic Chemical Reactions Types Synthesis Decomposition Single Replacement And Double R Chemical Reactions Ap Chemistry Reaction Types

Chemistry Worksheets Chemistry Lessons Teaching Chemistry

Synthesis Reactions Formation Reactions Module 5 Chemical Reactions Reactions Synthesis

Chemical Reaction Type Chart Reaction Types Chemical Reactions Teaching Chemistry

Types Of Chemical Reactions Doodle Notes Science Doodle Notes Doodle Notes Chemical Reactions Doodle Notes Science

Chemistry Reactions Combination Reaction When Two Or More Reactions Combine To Form Chemical Reactions Chemical Fun Science

Predicting Products For Decomposition Reactions Youtube Reactions Mo Co Chemistry

Predicting The Products Of Single And Double Displacement Reactions Teaching Chemistry Chemical Equation Interactive Science Notebook

This Set Of Chemical Reactions Pages Has A Page For Every Type Combustion Synthesis Decomposition Chemistry Lessons Chemistry Classroom Teaching Chemistry

Pin By Sabila On School Chemistry Classroom Chemical Reactions Chemistry Lessons
Comments
Post a Comment